ProductsDetails
Ursodeoxycholic acid (INN, BAN and AAN), also known as ursodiol (USAN) and the abbreviation UDCA, from the root-word for bear urso, as bear bile contains the substance, is one of the secondary bile acids, which are metabolic byproducts of intestinal bacteria.
ursodeoxycholic acid manufacturer tell about Ursodeoxycholic acid Endogenous effects
Primary bile acids are produced by the liver and stored in the gall bladder. When secreted into the intestine, primary bile acids can be metabolized into secondary bile acids by intestinal bacteria. Primary and secondary bile acids help the body digest fats. Ursodeoxycholic acid helps regulate cholesterol by reducing the rate at which the intestine absorbs cholesterol molecules while breaking up micelles containing cholesterol. Because of this property, ursodeoxycholic acid is used to treat (cholesterol) gallstones non-surgically. ursodeoxycholic acid manufacturer think that it is also used to relieve itching in pregnancy for some women who suffer obstetric cholestasis.
While some bile acids are known to be colon tumor promoters (e.g. deoxycholic acid), others such as ursodeoxycholic acid are thought to be chemopreventive, perhaps by inducing cellular differentiation and/or cellular senescence in colon epithelial cells.
It is believed to inhibit apoptosis.
Ursodeoxycholic acid has also been shown experimentally to suppress immune response such as immune cell phagocytosis. Prolonged exposure and/or increased quantities of systemic (throughout the body, not just in the digestive system) ursodeoxycholic acid can be toxic.
ursodeoxycholic acid manufacturer tell about Ursodeoxycholic acid Medical uses
A Cochrane review looking at primary biliary cirrhosis found that although ursodeoxycholic acid showed a reduction in liver biochemistry, jaundice, and ascites, it did not decrease mortality or liver transplantation. Ursodiol is the only FDA approved drug to treat primary biliary cirrhosis.
Ursodiol may be used for biliary stasis in pregnant women to relieve the symptoms of itching and decrease bile absorption.
In absence of biochemical response to ursodeoxycholic acid in PBC, its use is associated with an incidence of 20% hepatocellular carcinoma in 15 years.
In children, ursodeoxycholic acid use is not licensed, as its safety and effectiveness have not been established. ursodeoxycholic acid manufacturer think that Evidence is accumulating that ursodeoxycholic acid is ineffective and unsafe in neonatal hepatitis and neonatal cholestasis.
There is insufficient evidence to justify routine use of ursodeoxycholic acid in cystic fibrosis, especially that available data for analysis of long-term outcomes such as death or need for liver transplantation is lacking.
In double the recommended daily dose ursodeoxycholic acid reduces elevated liver enzyme levels in those with primary sclerosing cholangitis, but its use was associated with an increased risk of serious adverse events (the development of cirrhosis, varices, death or liver transplantation) in patients who received ursodeoxycholic acid compared with those who received placebo. ursodeoxycholic acid manufacturer think that Serious adverse events, were more common in the ursodeoxycholic acid group than the placebo group. The risk was 2.1 times greater for death, transplantation, or minimal listing criteria in patients on ursodeoxycholic acid than for those on placebo.
It is concluded that ursodeoxycholic acid use is associated with improved serum liver tests that do not always correlate with improved liver disease status. WHO Drug Information advises against its use in primary sclerosing cholangitis in unapproved doses beyond 13–15 mg/kg/day.
Ursodeoxycholic acid Mechanism of action
The drug reduces cholesterol absorption and is used to dissolve (cholesterol) gallstones in patients who want an alternative to surgery. If the patient stops taking the drug the gallstones tend to recur if the condition that gave rise to their formation does not change. For these reasons, it has not supplanted surgical treatment by cholecystectomy.
Also used to relieve itching in intrahepatic cholestasis of pregnancy (naltrexone may also be used).
ursodeoxycholic acid manufacturer tell about Ursodeoxycholic acid Trade names
Ursodeoxycholic acid can be chemically synthesized and is marketed under multiple trade names, including Actigall, BILIVER, Coric, Deursil, Egyurso, Udiliv, UDOXYL, Urso, Urso Forte, Ursocol, Ursofalk, Ursosan, Ursoserinox and Udimarin (India)
ursodeoxycholic acid manufacturer tell about ursodeoxycholic acid Chemistry
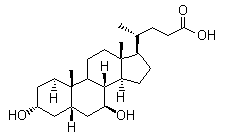
Molecule structure of Ursodeoxycholic acid (CAS NO.128-13-2):
IUPAC Name: (4R)-4-[(3R,5S,7S,8R,9S,10S,13R,14S,17R)-3,7-Dihydroxy-10,13-dimethyl-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl]pentanoic acid
Molecular Weight: 392.572 g/mol
Molecular Formula: C24H40O4
Density: 1.128 g/cm3
Melting Point: 203-204 °C(lit.)
Boiling Point: 547.1 °C at 760 mmHg
Flash Point: 298.8 °C
Index of Refraction: 1.543
Molar Refractivity: 109.65 cm3
Molar Volume: 347.8 cm3
Surface Tension: 46 dyne/cm
Enthalpy of Vaporization: 95.01 kJ/mol
Vapour Pressure: 2.98E-14 mmHg at 25 °C
Solubility Ethanol: 50 mg/mL, clear
Water Solubility: practically insoluble
XLogP3-AA: 4.9
H-Bond Donor: 3
H-Bond Acceptor: 4
Rotatable Bond Count: 4
Exact Mass: 392.29266
MonoIsotopic Mass: 392.29266
Topological Polar Surface Area: 77.8
Heavy Atom Count: 28
Canonical SMILES: CC(CCC(=O)O)C1CCC2C1(CCC3C2C(CC4C3(CCC(C4)O)C)O)C
Isomeric SMILES: C[C@H](CCC(=O)O)[C@H]1CC[C@@H]2[C@@]1(CC[C@H]3[C@H]2[C@H](C[C@H]4C@@]3(CC[C@H](C4)O)C)O)C
InChI: InChI=1S/C24H40O4/c1-14(4-7-21(27)28)17-5-6-18-22-19(9-11-24(17,18)3)23(2)10-8-16(25)12-15(23)13-20(22)26/h14-20,22,25-26H,4-13H2,1-3H3,(H,27,28)/t14-,15+,16-,17-,18+,19+,20+,22+,23+,24-/m1/s1
InChIKey: RUDATBOHQWOJDD-UZVSRGJWSA-N
EINECS: 204-879-3
Product Categories: Bile Acids; Biochemistry; Steroids; Intermediates & Fine Chemicals; Pharmaceuticals; API's
ursodeoxycholic acid manufacturer tell about ursodeoxycholic acid Uses
Ursodeoxycholic acid (CAS NO.128-13-2) can be used as an anticholelithogenic
Ursodeoxycholic acid Production
Ursodeoxycholic acid is found in large quantities in bear bile
Ursodeoxycholic acid Toxicity Data With Reference
1.
mmo-sat 40 mg/L
MUREAV Mutation Research. 158 (1985),45.
2.
orl-rat TDLo:11,900 mg/kg (4-20D preg):TER
AIPTAK Archives Internationales de Pharmacodynamie et de Therapie. 246 (1980),149.
3.
orl-rat LD50:4600 mg/kg
BCFAAI Bollettino Chimico Farmaceutico. 126 (1987),282.
4.
ipr-rat LD50:890 mg/kg
NIIRDN “Drugs in Japan. Ethical Drugs, 6th Edition 1982“ Edited by Japan Pharmaceutical Information Center. 6 (1982),95.
5.
ivn-rat LD50:310 mg/kg
NIIRDN “Drugs in Japan. Ethical Drugs, 6th Edition 1982“ Edited by Japan Pharmaceutical Information Center. 6 (1982),95.
6.
ivn-mus LD50:240 mg/kg
NIIRDN “Drugs in Japan. Ethical Drugs, 6th Edition 1982“ Edited by Japan Pharmaceutical Information Center. 6 (1982),95.
ursodeoxycholic acid manufacturer tell about ursodeoxycholic acid Safety Profile
Hazard Codes: IrritantXi
Risk Statements: 36/37/38
R36/37/38:Irritating to eyes, respiratory system and skin.
Safety Statements: 24/25-36-26
S24/25:Avoid contact with skin and eyes.
S36:Wear suitable protective clothing.
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
WGK Germany: 2
RTECS: FZ2000000
F: 10
Poison by intraperitoneal and intravenous routes. Experimental teratogenic and reproductive effects. Mutation data reported. Stimulates the flow of bile to the duodenum (a cholagogic). When heated to decomposition it emits acrid smoke and irritating fumes.
ursodeoxycholic acid manufacturer tell about ursodeoxycholic acid Specification
Ursodeoxycholic acid (CAS NO.128-13-2) is also named as 17-beta-(1-Methyl-3-carboxypropyl)etiocholane-3-alpha,7-beta-diol ; 3,7-Dihydroxycholan-24-oic acid ; 3-alpha,7-beta-Dihydroxy-5-beta-cholanoic acid ; 3-alpha,7-beta-Dihydroxycholanic acid ; 3-alpha,7-beta-Dioxycholanic acid ; 3alpha,7beta-Dihydroxy-5beta-cholan-24-oic acid ; 4-10-00-01604 (Beilstein Handbook Reference) ; 7-beta-Hydroxylithocholic acid ; Actigall ; Arsacol ; BRN 3219888 ; CCRIS 5502 ; Cholit-ursan ; Delursan ; Destolit ; Deursil ; Litursol ; Lyeton ; NSC 657950 ; NSC 683769 ; Peptarom ; Solutrat ; UNII-724L30Y2QR ; UrSO ; Ursacol ; Urso 250 ; Urso DS ; Urso Forte ; Ursobilin ; Ursochol ; Ursocholic acid, deoxy- ; Ursodamor ; Ursodeoxycholate ; Ursodiol ; Ursofalk ; Ursolvan . Ursodeoxycholic acid (CAS NO.128-13-2) is white crystalline powder.
1. Name of the medicinal product
Urdox 300mg Film-Coated Tablets, Ursodeoxycholic Acid 300mg Film-Coated Tablets
2. Qualitative and quantitative composition
Ursodeoxycholic acid 300mg.
Excipients: lactose.
For a full list of excipients, see section 6.1
3. Pharmaceutical form
Film coated tablets
Urdox tablets are white. film coated, convex tablets with "URDOX" on one side.
4. Clinical particulars
4.1 Therapeutic indications
Urdox tablets are indicated in the treatment of primary biliary cirrhosis (PBC) and for the dissolution of small to medium sized radiolucent, cholesterol-rich gall-stones in patients with a functioning gall bladder.
Cholesterol stones coated with calcium or stones composed of bile pigments are not dissolved by ursodeoxycholic acid. ursodeoxycholic acid manufacturer think that Urdox has a particular place in the treatment of patients in whom surgery is contraindicated or who are anxious to avoid surgery.
Paediatric population
Hepatobiliar disorder associated with cystic fibrosis in children aged 6 years to less than 18 years.
4.2 Posology and method of administration
Urdox tablets are for oral administration
To be taken with a drink of water.
Primary Biliary Cirrhosis
Adults and Elderly: 10 - 15mg ursodeoxycholic acid (UDCA) per kg per day in two to four divided doses.
Children: Dosage should be related to bodyweight.
Dissolution of gallstones
Adults and Elderly:
The usual dose is 6 - 12mg/kg/day either as a single night time dose or in divided doses. This may be increased to 15mg/kg/day in obese patients, if necessary.
The duration of treatment may be up to two years, depending on the size of the stone(s), and should be continued for three months after the apparent dissolution of the stone(s).
Children: Dosage should be related to bodyweight.
Paediatric population
Children with cystic fibrosis aged 6 year to less than 18 years: 20 mg/kg/day in 2-3 divided doses, with further increase to 30 mg/kg/day if necessary.
4.3 Contraindications
Ursodeoxycholic acid should not be used in patients:
1. with radio-opaque calcified gall-stones,
2. with acute inflammation of the gall bladder or biliary tract.
3. with occlusion of the biliary tract (occlusion of the common bile duct or a cystic duct)
4. with frequent episodes of biliary colic
5. with impaired contractability of the gall bladder
6. with hypersensitivity to bile acids or any excipient of the formulation
7. who are pregnant or breastfeeding, or in women who may become pregnant.
8. with chronic liver disease, peptic ulcers or in those with inflammatory diseases of the small intestine and colon.
Paediatric population
Unsuccessful portoenterostomy or without recovery of good bile flow in children with biliary atresia
4.4 Special warnings and precautions for use
Ursodeoxycholic acid should be taken under medical supervision.
During the first 3 months of treatment, the liver function parameters AST (SGOT), ALT (SGPT) and γ-GT should be monitored by the physician every 4 weeks, thereafter every 3 months. ursodeoxycholic acid manufacturer think that Apart from allowing for identification of responders and non-responders in patients being treated for primary biliary cirrhosis, this monitoring would also enable early detection of potential hepatic deterioration, particularly in patients with advanced stage primary biliary cirrhosis.
When used for the dissolution of cholesterol gallstones:
In order to assess therapeutic progress and for timely detection of any calcification of the gallstones, depending on stone size, the gall bladder should be visualised (oral cholecystography) with overview and occlusion views in standing and supine positions (ultrasound control) 6-10 months after the beginning of treatment.
If the gall bladder cannot be visualised on X-ray images, or in cases of calcified gallstones, impaired contractility of the gall bladder or frequent episodes of biliary colic, ursodeoxycholic acid should not be used.
When used for treatment of advanced stage of primary biliary cirrhosis:
In very rare cases decompensation of hepatic cirrhosis has been observed, which partially regressed after the treatment was discontinued.
If diarrhoea occurs, the dose must be reduced and in cases of persistent diarrhoea, the therapy should be discontinued.
Patients with rare hereditary problems of galactose intolerance, the Lapp lactose deficiency or glucose-galactose malabsorption should not take this medicine.
4.5 Interaction with other medicinal products and other forms of interaction
Ursodeoxycholic acid should not be administered concomitantly with charcoal, colestyramine, colestipol or antacids containing aluminium hydroxide and/or smectite (aluminium oxide), because these preparations bind ursodeoxycholic acid in the intestine and thereby inhibit its absorption and efficacy. Should the use of a preparation containing one of these substances be necessary, ursodeoxycholic acid manufacturer think that it must be taken at least 2 hours before or after ursodeoxycholic acid.
Ursodeoxycholic acid can increase the absorption of ciclosporin from the intestine. In patients receiving ciclosporin treatment, blood concentrations of this substance should therefore be checked by the physician and the ciclosporin dose adjusted if necessary.
In isolated cases ursodeoxycholic acid can reduce the absorption of ciprofloxacin.
Ursodeoxycholic acid has been shown to reduce the plasma peak concentrations (Cmax) and the area under the curve (AUC) of the calcium antagonist nitrendipine. An interaction with a reduction of the therapeutic effect of dapsone was also reported. These observations together with in vitro findings could indicate a potential for ursodeoxycholic acid to induce cytochrome P450 3A enzymes. Controlled clinical trials have shown, however, that ursodeoxycholic acid does not have a relevant inductive effect on cytochrome P450 3A enzymes.
Oral contraceptives, oestrogenic hormones and blood cholesterol lowering agents such as clofibrate may increase biliary lithiasis, which is a counter-effect to ursodeoxycholic acid used for dissolution of gallstones.
4.6 Pregnancy and lactation
There are no adequate data on the use of ursodeoxycholic acid, particularly in the first trimester of pregnancy. ursodeoxycholic acid manufacturer think that Animal studies have provided evidence of a teratogenic effect during the early phase of gestation. Ursodeoxycholic acid must not be used during pregnancy.Treatment should be discontinued immediately if pregnancy occurs and medical advice sought.
Women of childbearing potential should be treated only if they are using reliable contraception: non-hormonal or low-oestrogen oral contraceptive measures are recommended. However, in patients taking ursodeoxycholic acid for dissolution of gallstones, effective non-hormonal contraception should be used, since hormonal oral contraceptives may increase biliary lithiasis. ursodeoxycholic acid manufacturer think that The possibility of a pregnancy must be excluded before beginning treatment.
It is not known whether ursodeoxycholic acid passes into breast milk. Therefore, ursodeoxycholic acid should not be taken during lactation. If treatment with ursodeoxycholic acid is necessary, breastfeeding must be discontinued.
4.7 Effects on ability to drive and use machines
No effects on ability to drive and use machines have been observed.
4.8 Undesirable effects
The evaluation of undesirable effects is based on the following frequency data:
Very common (≥ 1/10)
Common (≥ 1/100 to < 1/10)
Uncommon (≥ 1/1,000 to < 1/100)
Rare (≥ 1/10,000 to < 1/1,000)
Very rare / Not known (< 1/10,000 / cannot be estimated from available data)
Gastrointestinal disorders:
In clinical trials, reports of pasty stools or diarrhoea during ursodeoxycholic acid therapy were common.
Very rarely, severe right upper abdominal pain has occurred during the treatment of primary biliary cirrhosis.
Ursodeoxycholic acid may give rise to nausea and vomiting. The frequency of these effects are not known.
Hepatobiliary disorders:
During treatment with ursodeoxycholic acid, calcification of gallstones can occur in very rare cases making them unable to be dissolved by bile acid therapy and resulting in surgery for some patients.
During therapy of the advanced stages of primary biliary cirrhosis, in very rare cases decompensation of hepatic cirrhosis has been observed, which partially regressed after the treatment was discontinued.
Skin and subcutaneaous disorders:
Very rarely, urticaria can occur.
Ursodeoxycholic acid may give rise to pruritus. The frequency of this effect is not known.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. ursodeoxycholic acid manufacturer think that Healthcare professionals are asked to report any suspected adverse reactions via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard.
4.9 Overdose
Diarrhoea may occur in cases of overdose. In general, other symptoms of overdose are unlikely because the absorption of ursodeoxycholic acid decreases with increasing dose and therefore more is excreted with the faeces.
No specific counter-measures are necessary and the consequences of diarrhoea should be treated symptomatically with restoration of fluid and electrolyte balance.
5. Pharmacological properties
5.1 Pharmacodynamic properties
When given by mouth, ursodeoxycholic acid reduces the ratio of cholesterol to bile salts plus phospholipids in bile, causing desaturation of cholesterol saturated bile. The exact mechanism of action has not been fully elucidated.
Paediatric population
Cystic fibrosis
From clinical reports long-term experience up to 10 years and more is available with UCDA treatment in paediatric patients suffering from cystic fibrosis associated hepatobiliary disorders (CFAHD). ursodeoxycholic acid manufacturer think that There is evidence that treatment with UCDA can decrease bile duct proliferation, halt progression of histological damage and even reverse hepato-biliary changes if given at early stage of CFAHD. Treatment with UDCA should be started as soon as the diagnosis of CFAHD is made in order to optimize treatment effectiveness.
5.2 Pharmacokinetic properties
Ursodeoxycholic acid is absorbed from the gastro-intestinal tract and undergoes first pass metabolism and enterohepatic recycling. It is partially conjugated in the liver before being excreted into bile and undergoing 7-α-dehydroxylation to lithocholic acid, some of which is excreted directly in the faeces. The rest is absorbed and mainly conjugated and sulphated by the liver before excretion in the faeces.
ursodeoxycholic acid manufacturer tell about ursodeoxycholic acid
Gallstones cause problems such as pain, jaundice (yellowing of your skin and the whites of your eyes), pancreatitis (inflammation of your pancreas), and gallbladder inflammation. They occur when bile, which is normally fluid, forms stones. Gallstones commonly contain lumps of cholesterol-like (fatty) material that has solidified and hardened. ursodeoxycholic acid manufacturer think that They may also contain bile pigments and calcium deposits. These stones can block the bile duct, causing pain.
Surgery is the usual treatment for gallstones that are causing symptoms, but treatment with ursodeoxycholic acid may dissolve smaller stones which are made mainly of cholesterol. ursodeoxycholic acid manufacturer think that It works by reducing the amount of cholesterol released by your liver into bile and by slowly dispersing the cholesterol. This breaks up the stones.
Some ursodeoxycholic acid preparations can also help to treat primary biliary cirrhosis. This is a condition that slowly damages the bile ducts in the liver, and as the disease progresses, it can damage the liver.
ursodeoxycholic acid manufacture tell about Before taking ursodeoxycholic acid
Some medicines are not suitable for people with certain conditions, and sometimes a medicine may only be used if extra care is taken. For these reasons, before you start taking ursodeoxycholic acid it is important that your doctor or pharmacist knows:
If you have problems with the way your liver works.
If you have a problem with your gallbladder other than gallstones.
If you have a condition which affects your intestines (such as Crohn's disease or ulcerative colitis), or if you have had surgery on your bowel.
If you are pregnant, trying for a baby or breast-feeding.
If you are taking other medicines, including those available to buy without a prescription, herbal or complementary medicines. In particular, let your doctor know if you are taking the oral contraceptive pill or hormone replacement therapy.
If you have ever had an allergic reaction to a medicine.
ursodeoxycholic acid manufacturer tell about How to take ursodeoxycholic acid
Before you start this treatment, read the manufacturer's printed information leaflet from inside your pack. ursodeoxycholic acid manufacturer think that The leaflet will give you more information about the specific brand of ursodeoxycholic acid you have been given, and a full list of side-effects which you may experience from taking it.
Take ursodeoxycholic acid exactly as your doctor tells you to. How much you need to take will depend upon what you are being treated for. Your doctor will tell you exactly how much to take and when, and the dosage directions will be on the label of the pack to remind you. To dissolve gallstones, it is usually taken once daily at bedtime. Sometimes your doctor may suggest you take two doses each day, in which case, take your last dose of the day at bedtime. ursodeoxycholic acid manufacturer think that If you are taking ursodeoxycholic acid for primary biliary cirrhosis, it is likely that you will be asked to take 2-4 doses daily.
Try to take ursodeoxycholic acid at the same times each day as this will help you to remember to take it. Take each of your doses with a snack or just after eating a meal.
If you are taking ursodeoxycholic acid liquid medicine, make sure you shake the bottle well before you pour out a dose.
If you forget to take a dose, take one as soon as you remember unless it is nearly time for your next dose, in which case skip the missed dose. Do not take two doses at the same time to make up.
Getting the most from your treatment
Try to keep your regular appointments with your doctor. ursodeoxycholic acid manufacturer think that This is so your doctor can check on your progress.You will need to have blood tests, scans or X-rays from time to time.
It is important that you follow any dietary advice that you have been given by your doctor. Try to avoid foods that are high in calories or cholesterol.
You may need to take ursodeoxycholic acid for up to two years for the treatment of gallstones. Once the gallstones have dissolved, your doctor may continue your treatment for three to four months to ensure that they have completely cleared up. Treatment for primary biliary cirrhosis is usually long-term unless you experience an adverse effect.
If you buy any medicines, check with a pharmacist that they are suitable to take with this medicine. ursodeoxycholic acid manufacturer think that This is particularly important if you are buying indigestion remedies. Some antacid preparations contain aluminium salts which can interfere with the way ursodeoxycholic acid works.
If you are using oral combined hormonal contraception (the 'pill'), please ask your doctor for advice. A method of contraception that contains less oestrogen may be more suitable for you.
Can ursodeoxycholic acid cause problems?
Along with their useful effects, most medicines can cause unwanted side-effects although not everyone experiences them. ursodeoxycholic acid manufacturer think that These usually improve as your body adjusts to the new medicine, but speak with your doctor or pharmacist if any of the following side-effects continue or become troublesome.
If you experience any other symptoms which you think may be due to this medicine, speak with your doctor or pharmacist.
ursodeoxycholic acid manufacturer tell about How to store ursodeoxycholic acid
Keep all medicines out of the reach and sight of children.
Store in a cool, dry place, away from direct heat and light.